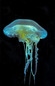
Chrysaora quinquecirrhaSea nettle
Geographic Range
Chrysaora quinquecirrha (Desor, 1848), commonly known as the Atlantic sea nettle or East Coast sea nettle, is broadly dispersed in tepid waters along the coasts of the Atlantic and Indian Oceans, as well as the Western Pacific. Along the United States east coast this species is common to abundant from southern New England to as far south as Brazil. In Virginia waters these jellies first appear in May, and usually vanish around September, though some occasionally remain well into November. (Calder, 1972)
- Biogeographic Regions
- indian ocean
- atlantic ocean
- pacific ocean
Habitat
Chrysaora quinquecirrha inhabits temperate waters and Atlantic estuaries which are low in salinity. In the Chesapeake Bay, from July through August, the nettles are most commonly found in meso-and polyhaline waters of the copious creeks and rivers. (Calder, 1972)
- Habitat Regions
- temperate
- tropical
- saltwater or marine
- Aquatic Biomes
- coastal
- brackish water
- Other Habitat Features
- estuarine
- intertidal or littoral
Physical Description
The body of Chrysaora quinquecirrha is mainly composed of an outer epidermis cup, an inner gastrodermis layer, and tentacles. Along the outside of the rim of the umbrella cup are long, skinny tentacles, which may grow up to 50 centimeters. The dome-shaped body of the jellyfish is approximately 25 centimeters in width, and has 8 scalloped, flower-petal shaped lobes from which tentacles extend. Each octant bears around 7 to 10 tentacles, all of which are lined with nematocysts (specialized stinging organelles). Four long, ribbon-like oral arms extend from the middle of the umbrella. The arms bring food up to the mouth, which is the only opening comprising the digestive system. This opening is lined with thousands of small mouthlet pores. The adult Atlantic sea nettle’s body is an opaque white color, often with red streaks or dots visible through the cup and tentacles. The life of C. quinquecirrha is dominated by two main cycles, each with a distinct body plan. First, the jellyfish live as a sessile polyp, then as a mobile medusa. The polyp stage is characterized by strobilation, in which the segmented polyp asexually produces young medusa. The medusa is the second stage of the life cycle. (Calder, 1972; Costello, et al., 2008)
- Other Physical Features
- ectothermic
- heterothermic
- radial symmetry
- polymorphic
- venomous
- Sexual Dimorphism
- sexes alike
-
- Average length
- 50 cm
- 19.69 in
-
- Average wingspan
- 25 cm
- 9.84 in
Development
Chrysaora quinquecirrha has two different body forms during its life cycle. The first form is a polypoid stage, where the organism is a small and sessile stalk, generally only millimeters long. Tentacles facilitate feeding. The polyp may either remain sessile, resembling coral and sea anemones, or it may be free-floating. Due to the polyp’s ability to bud asexually, it can either remain solitary or be colonial. Polyp strobilation, or budding, may lead to the appearance of ephyra, which are small, immature jellies. (Calder, 1972)
From ephyra to adult medusa, C. quinquecirrha has six different stages. These stages are categorized by the change in morphological structure. Two stages involve the growth of the ephyra, while the other four stages are for medusa development. The first four stages seen in species growth have been reproduced in the laboratory, while the last two stages have been recorded from nature. Stage I consists of newly-liberated ephyra, from polyps, which average between two to three and a half millimeters wide from lappet-tip to lappet-tip across the tiny medusa. Stage II is characterized by the presence of primary tentacles, and the development of the oral arms. As the medusa enters stage III, the lappets tend to fold under the medusa, thereby reducing its resemblance to its ephyra stage. Stage IV development is noted by the appearance of secondary tentacles between the primary tentacles. Stage V introduces the growth of 16 tertiary tentacles in the medusa. At this point there are 40 tentacles, and 48 lappets. The last stage, stage VI, is when the medusa has grown to a size of seven or more tentacles and eight or more lappets per octant. As previously mentioned, these last two stages have not been successfully reproduced in the lab, but research has shown that tentacle numbers in adult medusa vary, and are not a dependable taxonomic character in this group of Schyphozoa. (Calder, 1972)
- Development - Life Cycle
- metamorphosis
- colonial growth
Reproduction
What attacts or induces the Atlantic sea nettle to reproduce is known. This species reproduces both sexually and asexually. (Cargo and Rabenold, 1978; Littleford, 1939)
- Mating System
- polygynandrous (promiscuous)
In their polyp form, Chrysaora quinquecirrha reproduces asexually. This is done through a variety of ways: strobilation, cyst production, and by changing polyp position through the use of stolons. Medusae are able to reproduce sexually. Females catch the sperm released into the water from the mouths of the males. The eggs, which are also held in the mouth, become fertilized, and remain attached to the female's oral arms. As the fertilized eggs develop, they grow into planula. These planula have a flattened, bean shape. Once the polyps develop fully into flower-shaped progeny, they are released into the ocean where they settle, and begin asexual reproduction. The polyp buds to produce identical copies of themselves, and eventually detach to be released into the ocean where it will undergo metamorphosis to the medusa stage. (Cargo and Rabenold, 1978; Littleford, 1939)
- Key Reproductive Features
- seasonal breeding
- gonochoric/gonochoristic/dioecious (sexes separate)
- sexual
- asexual
- fertilization
- broadcast (group) spawning
- ovoviviparous
-
- Breeding season
- This species breeds during the summer months.
-
- Range time to independence
- 15 (low) hours
-
- Average time to independence
- 20 hours
Fertilized eggs will remain attached to the female parent's oral arms. The eggs into planula on the arms. Once the polyps develop fully into flower-shaped progeny, they are released into the ocean where they settle. (Cargo and Rabenold, 1978; Littleford, 1939)
- Parental Investment
- no parental involvement
- altricial
- female parental care
-
pre-fertilization
-
protecting
- female
-
protecting
-
pre-hatching/birth
-
protecting
- female
-
protecting
-
pre-weaning/fledging
-
protecting
- female
-
protecting
-
pre-independence
-
protecting
- female
-
protecting
Lifespan/Longevity
The lifespan of this species is unknown.
Behavior
Polyp stages of Chrysaora quinquecirrha can remain sessile or float freely. Asexual budding from sessile polyps can lead to colonial formation in the species. As medusa, the jellies move vertically and swim almost constantly. Vertical column distributions and swim patterns change in direct correlation with prey densities. This suggests hunting patterns are intrinsically linked to swimming. In-situ observations showed this species constantly swimming and propose it be considered a cruising predator. When observed in nature with minimal interference, the average time spent swimming within 24 hours was recorded from 90% and 100%. The swimming patterns witnessed were highly directional and non-random. With the ability to sense light and dark, medusae can determine their location and alignment in the water. The jellies also tend to swim against the current. As a result, they can become concentrated in large numbers, which make them coincidentally colonial.
One of the most visually noticeable behaviors of this species is the pulsation of the swimming bell. Pulsations are coordinated by nerve centers along the outside of the bell. Constant movements also facilitate oxygen exchange, which occurs over the jelly's entire body surface. (Costello, et al., 1998)
The disappearance of Atlantic sea nettles around the Chesapeake Bay area in winter months appears to be from their inability to swim away from the sea bottom. Cold water impedes and slows the jelly's ability to pulsate the swimming bell. This can lead to starvation, and usually concludes with large biomass deposits on the sediment surface. (Cargo and King, 1978; Sexton, et al., 2010)
Communication and Perception
Little information is known about communication in Chrysaora quinquecirrha, though it appears to be a colonial species. Some scyphozoans release and react to chemicals in the water during breeding seasons. Due to limited material on cnidarian nervous systems, how these chemicals are interpreted remains unclear. Scyphozoan nervous systems are usually comprised of a scattered net of cells, while some species display more organized nerve rings. In those species where nerve rings appear to be nonexistent the nerve cells form structures called rhopallia, arranged around the rim of the umbrella. Rhopalliums are typically associated with a pair of sensory pits, a balance organ for orientation, and sometimes pigment-cupped ocelli, or “eye spots.”
Commonly these eye-like structures are found in the medusa stage, even though polyps from all cnidarian classes are defined as light-sensitive. Photoreceptors of jellyfish are classified as the ciliary type, meaning one or more adapted cilia form the photoreceptive structure. Rhabdomeric photoreceptors are found in other invertebrate groups, whereas ciliary types are normally found in vertebrate eyes. Therefore, the photoreceptors of cnidarians may belong to the same evolutionary line as those of vertebrates. Extra ocular photosensitivity is prevalent throughout the cnidarians, with neurons, epithelial cells, and muscle cells facilitating light detection. (Barnes, 1974; Seymour, et al., 2003; Stierwald, 2004)
- Other Communication Modes
- pheromones
- Perception Channels
- polarized light
- tactile
- chemical
Food Habits
Atlantic sea nettles are carnivorous. The diet consists of zooplankton, ctenophores, as well as other jellies. Plankton (microscopic plants and animals drifting in the water) predominate the dietary regimen of the jelly. They tend to also prey upon small crustaceans, comb jellies, and fish eggs and larvae. Nettles also consume young minnows, bay anchovy eggs, worms, and mosquito larvae. Due to the large variety of prey, combined with their highly effective hunting style, C. quinquecirrha seldom goes without something to eat. (Cargo and Rabenold, 1978; Ford, et al., 1997; Purcell, 1992)
- Primary Diet
-
carnivore
- piscivore
- eats eggs
- eats non-insect arthropods
- vermivore
- eats other marine invertebrates
- planktivore
- Animal Foods
- fish
- eggs
- aquatic or marine worms
- aquatic crustaceans
- cnidarians
- zooplankton
Predation
Very few animals feed on these jellies since they are covered with stinging cells and have toxic venom. Sea turtles (mainly the leatherback turtle, Dermochelys coriacea), are possibly the best known predator of jellyfish and are regularly seen where jellyfish concentrations are high. Other fish, such as ocean sunfish also prey on jellyfish. One of the biggest predators of jellyfish are other jellyfish, mainly of other species.
In some Asian cultures, some fishermen routinely catch jellies to be dried and sold to restaurants. In Asia, jellies are seen as a delicacy. (Houghton, et al., 2006)
-
- Known Predators
-
- Leatherback turtle, Dermochelys coriacea
- Ocean sunfish, Mola mola
- Other jellyfish, Medusozoa
- Humans, Homo sapiens
Ecosystem Roles
The scyphomedusae blooms of Chrysaora quinquecirrha effect the aquatic ecosystem they inhabit. Because it effects trophic interactions within the food chain and the distribution of nutrients, it is considered a keystone species. Feeding on ctenophores, C. quinquecirrha eliminates the main predator of copepods, thus positively influencing their abundance. Not only does a higher concentration of copepods benefit planktonic populations, it also benefits fish species that prey on plankton. When these gelatinous creatures die, their bodies collect on the underwater sediment and through decomposition contribute to the carbon cycle. Though these bodies contribute to the carbon cycle, they also contribute to the successful increase of bacteria growth. (Baird and Ulanowicz, 1989; Purcell and Decker, 2005; Sexton, et al., 2010)
- Ecosystem Impact
- keystone species
Economic Importance for Humans: Positive
The diet of Chrysaora quinquecirrha has an indect effect on finfish and shellfish populations. By preying on ctenophores, populations of planktonic larvae flourish. These plankton are fed on by shellfish and finfish. Larger populations of the plankton result in larger populations of finfish and shellfish. (Purcell and Decker, 2005)
Economic Importance for Humans: Negative
Blooms of Chrysaora quinquecirrha negatively impact humans. Economically, these large masses of jellies deter swimmers, beach goers, and tourists from entering the water. If one comes in physical contact with the Atlantic sea nettle, thousands of stinging nematocysts on tentacles penetrate toxins into the skin, causing a painful rash.
- Negative Impacts
-
injures humans
- bites or stings
- venomous
Conservation Status
Chrysaora quinquecirrha populations are not under consideration for conservation status.
-
- IUCN Red List
- Not Evaluated
-
- US Federal List
- No special status
-
- CITES
- No special status
-
- State of Michigan List
- No special status
Contributors
Nate Lanier (author), Radford University, Gregory Zagursky (editor), Radford University.
Alexi Weber (author), Radford University, Renee Mulcrone (editor), Special Projects.
Glossary
- Atlantic Ocean
-
the body of water between Africa, Europe, the southern ocean (above 60 degrees south latitude), and the western hemisphere. It is the second largest ocean in the world after the Pacific Ocean.

- Pacific Ocean
-
body of water between the southern ocean (above 60 degrees south latitude), Australia, Asia, and the western hemisphere. This is the world's largest ocean, covering about 28% of the world's surface.

- altricial
-
young are born in a relatively underdeveloped state; they are unable to feed or care for themselves or locomote independently for a period of time after birth/hatching. In birds, naked and helpless after hatching.
- asexual
-
reproduction that is not sexual; that is, reproduction that does not include recombining the genotypes of two parents
- brackish water
-
areas with salty water, usually in coastal marshes and estuaries.
- carnivore
-
an animal that mainly eats meat
- chemical
-
uses smells or other chemicals to communicate
- coastal
-
the nearshore aquatic habitats near a coast, or shoreline.
- colonial
-
used loosely to describe any group of organisms living together or in close proximity to each other - for example nesting shorebirds that live in large colonies. More specifically refers to a group of organisms in which members act as specialized subunits (a continuous, modular society) - as in clonal organisms.
- colonial growth
-
animals that grow in groups of the same species, often refers to animals which are not mobile, such as corals.
- diurnal
-
- active during the day, 2. lasting for one day.
- ectothermic
-
animals which must use heat acquired from the environment and behavioral adaptations to regulate body temperature
- estuarine
-
an area where a freshwater river meets the ocean and tidal influences result in fluctuations in salinity.
- female parental care
-
parental care is carried out by females
- fertilization
-
union of egg and spermatozoan
- heterothermic
-
having a body temperature that fluctuates with that of the immediate environment; having no mechanism or a poorly developed mechanism for regulating internal body temperature.
- hibernation
-
the state that some animals enter during winter in which normal physiological processes are significantly reduced, thus lowering the animal's energy requirements. The act or condition of passing winter in a torpid or resting state, typically involving the abandonment of homoiothermy in mammals.
- internal fertilization
-
fertilization takes place within the female's body
- intertidal or littoral
-
the area of shoreline influenced mainly by the tides, between the highest and lowest reaches of the tide. An aquatic habitat.
- introduced
-
referring to animal species that have been transported to and established populations in regions outside of their natural range, usually through human action.
- keystone species
-
a species whose presence or absence strongly affects populations of other species in that area such that the extirpation of the keystone species in an area will result in the ultimate extirpation of many more species in that area (Example: sea otter).
- metamorphosis
-
A large change in the shape or structure of an animal that happens as the animal grows. In insects, "incomplete metamorphosis" is when young animals are similar to adults and change gradually into the adult form, and "complete metamorphosis" is when there is a profound change between larval and adult forms. Butterflies have complete metamorphosis, grasshoppers have incomplete metamorphosis.
- motile
-
having the capacity to move from one place to another.
- native range
-
the area in which the animal is naturally found, the region in which it is endemic.
- ovoviviparous
-
reproduction in which eggs develop within the maternal body without additional nourishment from the parent and hatch within the parent or immediately after laying.
- pheromones
-
chemicals released into air or water that are detected by and responded to by other animals of the same species
- piscivore
-
an animal that mainly eats fish
- planktivore
-
an animal that mainly eats plankton
- polarized light
-
light waves that are oriented in particular direction. For example, light reflected off of water has waves vibrating horizontally. Some animals, such as bees, can detect which way light is polarized and use that information. People cannot, unless they use special equipment.
- polygynandrous
-
the kind of polygamy in which a female pairs with several males, each of which also pairs with several different females.
- polymorphic
-
"many forms." A species is polymorphic if its individuals can be divided into two or more easily recognized groups, based on structure, color, or other similar characteristics. The term only applies when the distinct groups can be found in the same area; graded or clinal variation throughout the range of a species (e.g. a north-to-south decrease in size) is not polymorphism. Polymorphic characteristics may be inherited because the differences have a genetic basis, or they may be the result of environmental influences. We do not consider sexual differences (i.e. sexual dimorphism), seasonal changes (e.g. change in fur color), or age-related changes to be polymorphic. Polymorphism in a local population can be an adaptation to prevent density-dependent predation, where predators preferentially prey on the most common morph.
- radial symmetry
-
a form of body symmetry in which the parts of an animal are arranged concentrically around a central oral/aboral axis and more than one imaginary plane through this axis results in halves that are mirror-images of each other. Examples are cnidarians (Phylum Cnidaria, jellyfish, anemones, and corals).
- saltwater or marine
-
mainly lives in oceans, seas, or other bodies of salt water.
- seasonal breeding
-
breeding is confined to a particular season
- sessile
-
non-motile; permanently attached at the base.
Attached to substratum and moving little or not at all. Synapomorphy of the Anthozoa
- sexual
-
reproduction that includes combining the genetic contribution of two individuals, a male and a female
- social
-
associates with others of its species; forms social groups.
- solitary
-
lives alone
- tactile
-
uses touch to communicate
- temperate
-
that region of the Earth between 23.5 degrees North and 60 degrees North (between the Tropic of Cancer and the Arctic Circle) and between 23.5 degrees South and 60 degrees South (between the Tropic of Capricorn and the Antarctic Circle).
- tropical
-
the region of the earth that surrounds the equator, from 23.5 degrees north to 23.5 degrees south.
- venomous
-
an animal which has an organ capable of injecting a poisonous substance into a wound (for example, scorpions, jellyfish, and rattlesnakes).
- zooplankton
-
animal constituent of plankton; mainly small crustaceans and fish larvae. (Compare to phytoplankton.)
References
Baird, D., R. Ulanowicz. 1989. The seasonal dynamics of the Chesapeake Bay ecosystem. Ecological Monographs, 59 (4): 329-364.
Balamurugan, E., D. Kumar, V. Menon. 2009. Proapoptotic effect of Chrysaora quinquecirrha (sea nettle) nematocyst venom peptide in HEp 2 and HeLa Cells. European Journal of Scientific Research, 35 (3): 355-367. Accessed June 21, 2011 at http://www.eurojournals.com/ejsr_35_3_04.pdf.
Barnes, R. 1974. Invertebrate zoology. Philiadelphia: 3d ed. Saunders.
Calder, D. 1972. Development of the sea nettle Chrysaora quinquecirrha. Chesapeake Science, 13: 40-44.
Cargo, D., D. King. 1978. Forecasting the abundance of the sea nettle, Chrysaora quinquecirrha, in the Chesapeake Bay. Estuaries and Coasts. Estuaries and Coasts, 13: 485-491.
Cargo, D., G. Rabenold. 1978. Observations on the asexual reproductive activities of the sessile stages of the sea nettle Chrysaora quinquecirrha (Scyphozoa). Estuaries and Coasts, 3: 20-27.
Clifford, H., D. Cargo. 1987. Feeding rates of the sea nettle, Chrysaora quinquecirrha, under laboratory conditions. Estuaries and Coasts, 1: 58-61.
Condon, R., M. Decker, J. Purcell. 2001. Effects of low dissolved oxygen on survival and sexual reproduction of scyphozoan polyps (Chrysaora quinquecirrha). Hydrobiologia, 451: 89-95.
Costello, J., S. Colin, J. Dabiri. 2008. Medusan morphospace: phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebrate Biology, 127(3): 265-290.
Costello, J., E. Klos, M. Ford. 1998. In situ time budgets of the scyphomedusae Aurelia aurita, Cyanea sp, and Chrysaora quinquecirrha. J. Plankton Res, 20: 383-391.
Cowan, J., E. Houde. 1993. Relative predation potentials of scyphomedusae, ctenophores and planktivorous fish on ichthyoplankton in the Chesapeake Bay. Marine Ecology-Progress Series, 95: 55-65.
Feigenbaum, D., M. Kelly. 1984. Changes in the lower Chesapeake Bay food chain in presence of the sea nettle Chrysaora quinquecirrha (Scyphomedusa). Marine Ecology-Progress Series, 19: 39-47.
Ford, M., J. Costello, K. Heidelberg, J. Purcell. 1997. Swimming and feeding by the scyphomedusa Chrysaora quinquecirrha. Marine Biology, 129: 355-362.
Houghton, J., T. Doyle, M. Wilson, J. Davenport, G. Hays. 2006. Jellyfish aggregations and leatherback turtle foraging. Ecology, 87 (8): 1967-1972.
Littleford, R. 1939. The life cycle of Dactylometra quinquecirrha, L. agassiz in the Chesapeake Bay. The Biological Bulletin, 77: 368-381.
Lucy, J. 1983. Handle with care! Mid-Atlantic marine animals that demand your respect. Educational Series, 26: 22.
Purcell, J., M. Decker. 2005. Effects of climate on relative predation by scyphomedusae and ctenophores on copepods in Chesapeake Bay during 1987-2000. Limnology and Oceanography, 50 (1): 367-387.
Purcell, J. 1992. Effects of predation by the scyphomedusan Chrysaora quinquecirrha on zooplankton populations in Chesapeake Bay, USA. Marine Ecology-Progress Series, 87: 65-76.
Rice, N., A. Powell. 1970. Observations on three species of jellyfishes from Chesapeake Bay with special reference to their toxins. I. Chrysaora (Dactylometra) quinquecirrha. The Biological Bulletin, 198: 180-187.
Sexton, M., R. Hood, J. Sarkodee-ado, A. Liss. 2010. Response of Chrysaora quinquecirrha medusae to low temperature. Hydrobiologia, 645: 125-133.
Seymour, J., R. Wallèn, K. Nordström, D. Nilsson. 2003. A simple visual system without neurons in jellyfish. Proceedings: Biological Sciences, 270: 2349-2354.
Stierwald, M. 2004. On the evolution of cnidarian eyes. University of Basel, PhD Thesis: 1-54.